Diamond-Based Quantum Sensing Microscope Offers Effective Approach for Quantifying Cellular Forces
Cells rely on constant interplay and information exchange with their micro-environment to ensure their survival and perform biological functions. Hence, precise quantification of tiny cellular adhesion forces, spanning from piconewtons to a few nanonewtons, is crucial for understanding the intricacies of force modulation in cells.
Over the past few decades, various methods have been successfully developed for measuring cellular adhesive forces. Currently, several leading technologies such as traction force microscopy (TFM), optical/magnetic tweezers, and molecular tension-based fluorescence microscopy (MTFM) are widely used for measuring cellular forces.
However, these techniques have notable limitations in terms of sensitivity and data interpretation, which impede our ability to comprehensively understand mechanobiology. Additionally, the MTFM technique is hindered by the stochastic nature of fluorophore photobleaching.
Therefore, it is essential to develop a new technique that can accurately measure cell adhesive forces in a fluorescent label-free manner. This is crucial to advancing the field of mechanobiology.

A project led by Professor Zhiqin Chu from the Department of Electrical and Electronic Engineering at the University of Hong Kong (HKU) and Professor Qiang Wei from Sichuan University applied label-free quantum sensing technology to measure cellular force on a nanoscale. This overcomes the limitations of traditional cellular force apparatus and offers new insights into studying cellular mechanics, including the influence of cellular adhesion forces on cancer cell spreading.
The research team has developed a new Quantum-Enhanced Diamond Molecular Tension Microscopy (QDMTM) that offers an effective approach for studying cell adhesion forces. Compared to cell force measurement methods that utilize fluorescent probes, QDMTM has the potential to overcome challenges such as photobleaching, limited sensitivity, and ambiguity in data interpretation. Furthermore, QDMTM sensors can be cleaned and reused, enhancing the absolute accuracy of comparing cell adhesion forces across various samples.
The new method fundamentally changes the way for studying important issues such as cell–cell or cell–material interactions, with significant implications for biophysics and biomedical engineering. The findings have been published in Science Advances, in an article titled "Quantum-enhanced diamond molecular tension microscopy for quantifying cellular forces."
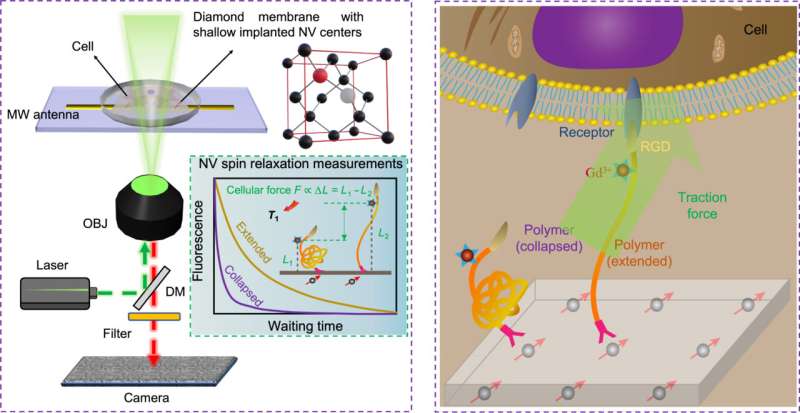
The research team developed QDMTM by combining the extension of polymer (acting as a force transducer) induced by cellular forces with the longitudinal relaxation time of NV. The unique quantum properties of NV center electron spins in diamond serve as the fundamental basis for the unprecedented sensitivity and precision of QDMTM.
The uniqueness of this innovation lies in the utilization of a "force transducer" which is a force-responsive polymer, capable of converting mechanical signals into magnetic signals. By measuring the changes in NV spin relaxation time caused by the magnetic noise, the adhesive forces exerted by cells on the "force transducer" can be determined. Existing measurement techniques are unable to effectively measure the stochastic magnetic signals at the nanoscale.
The innovative QDMTM technique offers an effective approach to studying cell adhesion forces. Through their study, researchers were able to successfully differentiate cells in various adhesion states and found that the magnitude of cellular forces in different cell regions aligned with the previous findings.
This suggests that the QDMTM method is capable of accurately measuring cell adhesion forces. The next phase of their research focuses on expanding the quantum sensor from bulk diamond to nanoscale diamond particles, which will allow for the measurement of cell forces in any direction.